


Access to Life-saving Medicines
Countries in the ASEAN region persistently face crucial challenges to ensure continuous access to medicines for the population. These include disruptions in the availability of some essential medicines, including those needed to treat infectious diseases. These medicines are often claimed to be “non- commercially viable”; hence, they are not manufactured and made available to the public readily. When there is a disrupted supply of these essential medicines, ASEAN Member States will have to look for alternative sources. When this happens, states are compelled to pay higher prices and risk purchasing products of low quality.
The cost of life-saving medicines and orphan drugs can be extraordinarily high because they are so rare and unprofitable that manufacturing them would need government assistance. The prices vary widely among Member States due to the limited supply. The same could be said about new life-saving patented medicines, such as targeted cancer therapies that most health systems in ASEAN can ill-afford.
Fundamentally, ensuring access to essential medicines is a key objective of all health systems and an integral component of improving universal health coverage (UHC). Despite global and national efforts to improve access and affordability of medicines, millions of people, particularly those from low- and middle-income countries, remain without full access to quality, safe, and efficacious medicines at affordable prices.
In the past decade, several emerging infectious diseases were reported in the ASEAN region, such as avian influenza A H5N1, influenza A H1N1 (2009), severe acute respiratory syndrome (SARS), Nipah virus, leptospirosis, among others. During a public health emergency or pandemic, the sudden spike in demand for medicines such as antivirals could cause drug shortages in the region. Member States also face significant challenges in determining drug supplies and developing strategies to build an emergency drug stockpile effectively (R.J.Coker, et. al, Emerging Infectious Diseases in Southeast Asia: Regional Challenges to Control, Lancet, 2011).
ASEAN’s Vision to Ensure Drug Security and Self-reliance
In line with the Sustainable Development Goals (SDGs), the ASEAN Post-2015 Health Development Agenda was initiated to achieve a healthy, caring, and sustainable ASEAN community through four health clusters. The health clusters highlight different health priority issues: promoting healthy lifestyles, responding to hazards and emerging threats, strengthening health systems, and access to care and ensuring food safety.
The ASEAN Health Cluster III (AHC3) Work Programme for 2016-2020 on Strengthening Health Systems and Access to Care focuses on strengthening the regional capabilities, capacities, and advocacy in health system development to increase access to safe, affordable, quality, and holistic health care. Under the work plan, the ASEAN Drug Security and Self-Reliance (ADSSR) project was proposed, and Malaysia was given the mandate as the lead country and focal point for the project. The overall objective of the ADSSR is for all Member States to work together and improve access to medicines by enhancing drug security and self-reliance in the region. The scope of the ADSSR includes all medicines for therapeutic use, including essential drugs, orphan drugs, antidotes, high-cost medicines, biological products, and medicines for emerging infectious diseases, pandemics, and neglected tropical diseases (ASEAN Health Cluster 3 Work Programme). A similar effort to ensure vaccine security in the region, known as ASEAN Vaccine Security and Self-Reliance (AVSSR), was assigned to Thailand as the lead country.
From 2018 to 2020, four key activities were conducted: the preparation of the concept note, conduct of the Baseline Pharmaceutical Situational Analysis Survey, supplementary information search, and focus group discussion among the Member State-representatives. The survey and supplementary information search were carried out as situational analysis to examine the laws, regulations, and policies pertaining to production, regulatory, distribution, procurement, pricing, medicines use practices, and the gaps and challenges in the 10 Member States. In November 2019, the Technical Meeting on Regional Collaborative Strategy for the ADSSR was held in Putrajaya, Malaysia to examine the feasibility of regional collaboration and to develop the collaborative strategies for the project. The technical meeting produced the proposed framework and draft action plans for the regional collaborative strategy.
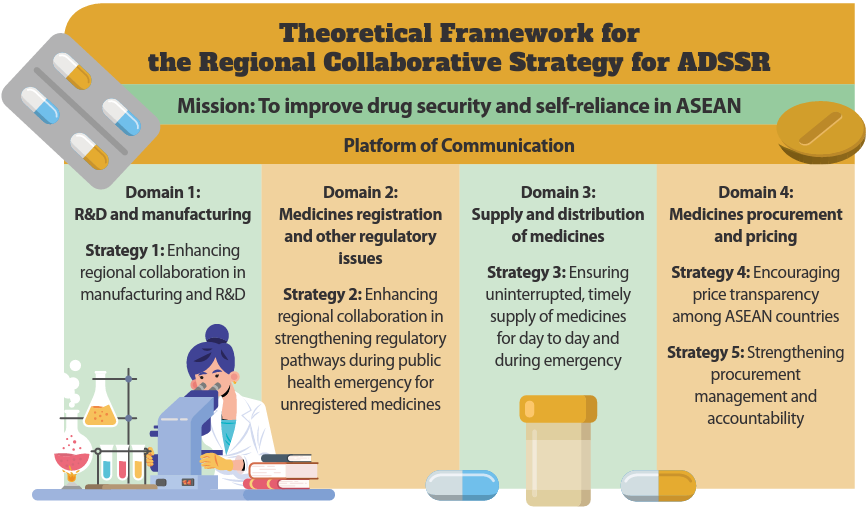
The proposed theoretical framework comprises four domains and five strategies. The domains are: (i) research and development (R&D) and manufacturing, (ii) medicine registration and other regulatory issues, (iii) supply and distribution of medicines, and (iv) medicine procurement and pricing.
Domain 1 addresses the growing demand in ASEAN’s pharmaceutical markets. It establishes regional collaboration among Member States in manufacturing and research and development. A good communication platform among the Member States is needed to share updates and promote the development of novel drugs especially for tropical diseases and non-commercially available medicines. This strategy is important to make ASEAN self-sufficient in drug manufacturing to fulfil the region’s own needs and demands.
Under domain 2, regional collaboration in strengthening regulatory pathways is highlighted. The alignment of regulatory pathways in all Member States is crucial to expedite the marketing authorisation approval without compromising the quality, safety and efficacy of medicines during a public health emergency. This will ensure the availability of medications during public health emergencies. The initiatives in this domain are crucial in support of the ASEAN Public Health Emergency Operation Centre.
The key strategy in the third domain of the ADSSR is to ensure the uninterrupted and timely supply of medicines. Enhancing collaboration among the Member States and strengthening the procurement capability are needed to ensure that medicines are available and in continuous supply in both normal times and emergencies.
The fourth domain lays out strategies to encourage price transparency among the Member States and to strengthen procurement management and accountability. Price information sharing is believed to be able to improve Member States’ negotiating power to ensure more affordable medicine prices for the country. Under this domain, the feasibility of different levels of medicines pooled procurement will also be explored.
Across all domains and strategies, effective cross-country communication is most critical, therefore sophisticated data exchange among the Member States will be instituted in the ADSSR action plans.
Overall, the regional collaborative strategy for ADSSR aims to improve drug security and self-reliance within the region, as well as to complement relevant pharmaceutical systems in Member States. As most Member States share similar issues with the access to non-commercially viable medicines, orphan medicines and expensive patented medicines, they are willing to participate in regional collaborations. They expect more competitive medicine pricing and the improved availability of more medicines in their countries.
Several countries have also expressed interest in collaboratively developing a regional pooled procurement mechanism. With economies of scale, pooled procurement may improve the affordability of drug prices significantly. The main rationale for pooled procurement is to increase the availability of affordable, quality essential pharmaceutical products in the market and the most desirable outcome is “value for money.” Although the idea of regional pooled procurement is still at its embryonic stage, the proposed regional collaborative strategies will lay down some important ground works towards it. For example, regulatory harmonisation, sharing of supplier information, and medicine price sharing are essential foundations to build pooled procurement in the region. Further engagements among all Member States to find common grounds and pursue political commitment are necessary to establish a feasible model of cooperation.
The baseline analysis identified many challenges faced by the Member States in the production, regulation, supply and distribution, procurement, and pricing of pharmaceuticals. The ADSSR framework draws out some feasible mechanisms to improve ASEAN’s self-reliance to ensure the availability of important medicines. As some ASEAN countries are pharmaceutical manufacturers and others are buyers, this creates the opportunities for collaboration in grouped-production or group-purchasing of non-commercially viable products from and for the ASEAN region, rather than sourcing from the other regions. It is also hoped that with ASEAN’s improved capacity in the production and procurement of medicines and active pharmaceutical ingredients (API), the region can be self- sufficient, rather than continually relying on and sourcing from the other regions.
To date, the regional collaborative strategy for ADSSR is still undergoing a consultation process. Once the regional collaborative strategy framework is endorsed, the lead country or institution for each initiative or project under the strategy, the expected outputs, and indicators, timelines, and resource needs will be further deliberated.
This initiative is still at its beginning stage. Malaysia, as the lead country, wishes to call for the continuous commitment from all Member States to address all the challenges towards achieving the objective of ADSSR. It is with great hope that the proposed strategies could help the ASEAN nations improve access to medicines and achieve better health outcomes for the population.